Practice Test: Chemistry (67)
Suggested Testing Time: 4 hours
To Take This Practice Test
- Use the answer key to record your responses.
- Prefer to take it offline? You can print the questions and answer key.
Remember:
- The practice test can give you a good indication of how you may perform on an actual test, but there is no guarantee that your results will be the same as on the actual test.
- The actual test looks and operates differently than this practice test. In addition, this test includes one or more assignments that allow you to handwrite and scan your responses. Review the Testing Tutorials and Demonstrations for more information about the actual test platform.
- A scientific calculator
 , periodic table
, periodic table  , and constants and formulas
, and constants and formulas  are provided on screen with your actual test.
are provided on screen with your actual test.
Question 1.
A teacher makes tennis balls, string, and tape available to students to simulate the Rutherford gold foil experiment. Which of the following student-designed activities would best demonstrate the observations that led to Rutherford's conclusions?
- Balls are placed equal distances apart on the floor to represent electrons dispersed throughout a uniform positive medium represented by the floor. The balls represent the gold particles that deflect the alpha particles.
- Several balls are clustered together on the floor to represent protons and neutrons in the gold atom nucleus. The nucleus is circled with multiple rings of string to represent the various orbitals' energy levels from which the electrons were ejected by the alpha particles.
- Balls are taped to the classroom floor about 2 m apart. Pieces of string are used to shape the outlines of s and p orbitals on the floor around the balls. Two small pieces of rolled-up tape are placed inside each orbital to represent electrons. The orbital shapes represent where the gold electrons are located when they interact with the alpha particles.
- Some balls are suspended about 2 m above the floor and 2 m apart in a straight line across the room to represent the gold atoms' nuclei in the foil. Students take turns throwing the other balls at the suspended balls and count the number of times a suspended ball is hit or missed. The hits represent deflection of the alpha particles by the gold nuclei.
Question 2.
Which of the following questions was addressed by Bohr's, but not by Rutherford's, atomic model?
- How do electrons transition between orbits to emit energy?
- Is there a distinction between a nucleus and the orbiting electrons?
- How do the positively charged protons hold on to the electrons?
- What is the specific location of the electrons in relation to the nucleus?
Question 3.
A student wants to make a poster that explains why gold has its particular atomic emission spectrum. The student decides to start with an explanation of a model of a gold atom. Which of the following types of models should the student use in the poster?
- quantum model
- planetary model
- nuclear model
- plum pudding model
Question 4.
Use the data below for a hypothetical element to answer the question that follows.
| Isotope | Atomic Mass | Percent Abundance |
|---|---|---|
| A H 24 | 23.98501417 | 78.99 |
| A H 25 | 24.98583696 | 10.00 |
| A H 26 | 25.98259292 | 11.01 |
What is the average atomic mass of the hypothetical element A H based on the data in the table?
- 23.99
- 24.31
- 24.98
- 25.00
Question 5.
Use the table below to answer the question that follows.
| Naturally Occurring Abundance (%) | Relative Masses ( atomic mass unit ) |
|---|---|
| 0.00550 | 234.041 |
| 0.720 | 235.044 |
| 99.275 | 238.051 |
A student is analyzing the data shown in the table about naturally occurring isotopes of an element. Based on the data, what is the average atomic mass of the element?
- 238.029 atomic mass unit
- 235.712 atomic mass unit
- 236.297 atomic mass unit
- 238.051 atomic mass unit
Question 6.
Which of the following diagrams shows the orbital notation for the ground state electron configuration of a neutral sulfur (S) atom?
-
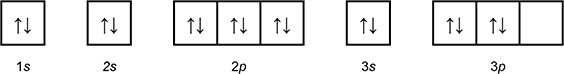 1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, the first 2 with an arrow pointing up and an arrow pointing down, the third blank, labeled 3p.
1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, the first 2 with an arrow pointing up and an arrow pointing down, the third blank, labeled 3p.
-
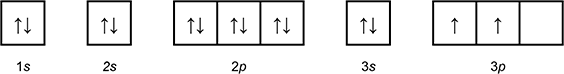 1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, the first 2 with an arrow pointing up, the third blank, labeled 3p.
1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, the first 2 with an arrow pointing up, the third blank, labeled 3p.
-
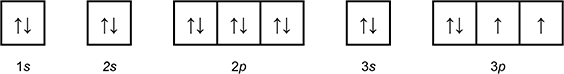 1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, the first box with an arrow pointing up and an arrow pointing down, the final 2 boxes with an arrow pointing up, labeled 3p.
1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, the first box with an arrow pointing up and an arrow pointing down, the final 2 boxes with an arrow pointing up, labeled 3p.
-
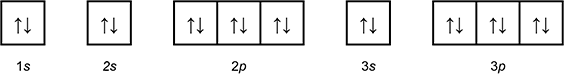 1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 3p.
1 box with an arrow pointing up, and an arrow pointing down labeled 1 s.
1 box with an arrow pointing up, and an arrow pointing down labeled 2 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 2p.
1 box with an arrow pointing up, and an arrow pointing down labeled 3 s.
3 connected boxes, each with an arrow pointing up and an arrow pointing down, labeled 3p.
Question 7.
Which of the following electron configurations represents a carbon atom in an excited state prior to hybridization?
- 1 s squared 2 s squared 2 p squared
- 1 s squared 2 s squared 2 p cubed
- 1 s squared 2 s to the first power 2 p squared
- 1 s squared 2 s to the first power 2 p cubed
Question 8.
Which of the following statements is true about the ions A l 3 +, N 3 negative, N a +, and O 2 negative ?
- A l 3 + has the largest ionic radius because it has the highest number of valence electrons repelling one another.
- N 3 negative has the largest ionic radius because it has the smallest number of protons attracting the electrons.
- N a + has the largest ionic radius because it has a full valence shell of electrons equally repelling each other.
- O 2 negative has the largest ionic radius because it has the highest number of neutrons increasing the attraction from the protons.
Question 9.
Which of the following is the correct electron configuration for a neutral iron ( F e ) atom?
- 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 4 s 2 4 d 6
- 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 4 s 2 3 d 6
- 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 4 s 2 4 p 6
- 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4 4 s 2 3 f 6
Question 10.
Which of the following compounds has a cation in the +3 oxidation state?
- carbon tetrachloride ( C C l 4 )
- magnesium sulfate ( M g S O 4 )
- sodium sulfide ( N a 2 S )
- aluminum oxide ( A l 2 O 3 )
Question 11.
Use the image below of a partial stock room label to answer the question that follows.
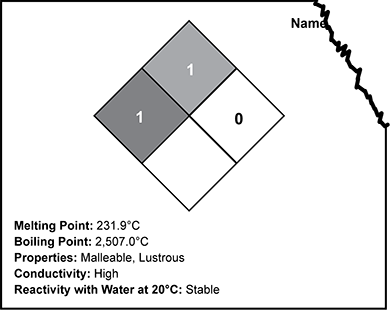
A diamond is subdivided into four smaller diamonds. The left, smaller diamond is dark gray and contains a 1. The top, smaller diamond is medium gray and contains a 1. The right, smaller diamond is light gray and contains a 0. The bottom, smaller diamond is white. Below the diamond is listed Melting Point 231.9 degrees Celsius, boiling point 2507 degrees Celsius, properties malleable and lustrous, conductivity high, and reactivity with water at 20 degrees Celsius stable.
The label of a stock element is damaged so that the name of the element is missing, as shown in the image above. The unknown element is hard and granular. Based on the data shown on the label, to which of the following elements could the label belong?
- tin (Sn)
- Sodium ( N a )
- sulfur (S)
- mercury (Hg)
Question 12.
Which of the following properties causes nitrogen to be more likely than boron to attract the electrons of another atom?
- Boron has a larger ionization energy.
- Nitrogen has a larger atomic radius.
- Boron has a higher number of electron shells.
- Nitrogen has a higher electronegativity.
Question 13.
Magnesium (Mg) has a first ionization energy of 738 kJ/mol and an atomic radius of about 136 pm. Which of the following pairs of values is most likely to be the first ionization energy and atomic radius of beryllium ( B e )?
- 496 kJ/mol; 73 pm
- 590 kJ/mol; 174 pm
- 899 kJ/mol; 90 pm
- 1,086 kJ/mol; 221 pm
Question 14.
Which of the following oxidation states is most likely to occur in an atom with a ground state electron configuration of 1 s 2 2 s 2 ?
- minus 2
- minus 1
- +1
- +2
Question 15.
As atomic number increases in a group of the periodic table, which of the following atomic properties decreases?
- electronegativity
- metallic character
- atomic mass
- atomic radius
Question 16.
What is the name of the compound with the formula CF4?
- carbon fluoride
- carbon tetrafluoride
- monocarbon tetrafluoride
- carbon( 4 ) fluoride
Question 17.
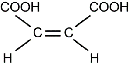
Use the skeletal structure below to answer the question that follows.
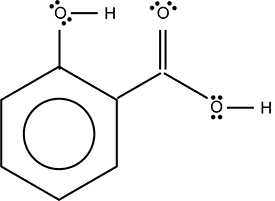
A circle is inscribed inside a larger hexagon. A line extends directly up from a vertex of the hexagon to an O surrounded by two pairs of dots and a line that extends to an H. Another line extends from the 2 o'clock vertex of the hexagon to another, dividing to form a double vertical line extending up and a single line extending down and to the right. The double line connects to an O surrounded by two pairs of dots. The single line connects to an O that is surrounded by two pairs of dots and a single line to an H.
Which of the following organic functional groups does the molecule represented by the skeletal structure contain?
- ketone
- ester
- carboxylic acid
- ether
Question 18.
What is the IUPAC name for the molecule c h 3 c h 2 c h c L c h 3 ?
- 2-chlorobutane
- 3-chloropropane
- 2-chloroethane
- 3-chloropentane
Question 19.
Use the table below to answer the question that follows.
| Compound | Number of Bonded Pairs | Number of Lone Pairs | Bond Angle |
|---|---|---|---|
| N H 3 | 3 | 1 | 107.8° |
| H 2 O | 2 | 2 | 104.5° |
| X e F 2 | 2 | 3 | 180.0° |
| C H 4 | 4 | 0 | 109.5° |
| P C l 5 | 5 | 0 | 90.0° |
The valence-shell electron-pair repulsion (VSEPR) model is used to help predict the shapes of molecules. Data regarding the actual arrangement of electrons and atoms in space are shown in the table. Which of the following claims about the VSEPR model can be supported by the patterns determined from the data?
- Compounds with only bonded pair electrons show no deviation from electron geometries.
- As the number of the lone pair electrons decreases, the angle of the bond decreases.
- The number of lone pair electrons determines the molecular geometry.
- The bond angle is equal to 360° divided by the number of bonded pairs.
Question 20.
Which of the following molecules has a bent molecular geometry?
- H 2 O
- C O 2
- N H 3
- C H 4
Question 21.
Use the Lewis structures below to answer the question that follows.
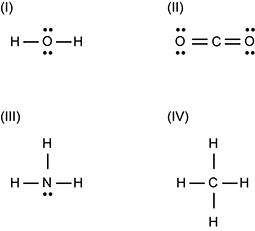
1 O is surrounded top and bottom by pairs of dots and left and right by single horizontal lines connected to H's. 2 C is connected to the left and to the right to double horizontal lines. Each pair of horizontal lines connects to an O that has a pair of dots to the top and another to the bottom. 3 N is surrounded to the top, left, and right by single horizontal lines connected to H's, and to the bottom by a pair of dots. 4 C is surrounded to the top, bottom, left, and right by single horizontal lines connected to H's.
Which of the Lewis structures shown illustrates a molecule with the greatest polarity?
- 1
- 2
- 3
- 4
Question 22.
Which of the following compounds is an ionic solid with a high melting point?
- magnesium sulfide
- silicon dioxide
- hydrogen chloride
- carbon tetrabromide
Question 23.
Which of the following diagrams correctly shows the ions in barium chloride?
-
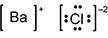 B a is surrounded by square brackets, followed by a superscripted plus sign. C l is surrounded by four pairs of dots and square brackets, followed by a superscripted negative sign 2.
B a is surrounded by square brackets, followed by a superscripted plus sign. C l is surrounded by four pairs of dots and square brackets, followed by a superscripted negative sign 2.
-
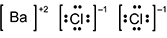 B a is surrounded by square brackets, followed by a superscripted plus 2. C l is surrounded by four pairs of dots and square brackets, followed by a superscripted negative 1. A second C l is also surrounded by four pairs of dots and square brackets, followed by a superscripted negative 1.
B a is surrounded by square brackets, followed by a superscripted plus 2. C l is surrounded by four pairs of dots and square brackets, followed by a superscripted negative 1. A second C l is also surrounded by four pairs of dots and square brackets, followed by a superscripted negative 1.
-
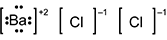 B a is surrounded by four pairs of dots and square brackets, followed by a superscripted plus 2. C l is surrounded by square brackets, followed by a superscripted negative 1. A second C l is also surrounded by square brackets, followed by a superscripted negative 1.
B a is surrounded by four pairs of dots and square brackets, followed by a superscripted plus 2. C l is surrounded by square brackets, followed by a superscripted negative 1. A second C l is also surrounded by square brackets, followed by a superscripted negative 1.
-
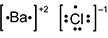 B a is surrounded by two dots and square brackets, followed by a superscripted plus 2. C l is surrounded by seven dots and square brackets, followed by a superscripted negative 1.
B a is surrounded by two dots and square brackets, followed by a superscripted plus 2. C l is surrounded by seven dots and square brackets, followed by a superscripted negative 1.
Question 24.
Which of the following molecules has the strongest O—H bond?
- H C l O 4
- H C l O 3
- H C l O 2
- H C l O
Use the information below to answer the two questions that follow.
In an experiment, colorless, transparent solution A is analyzed using the following procedure:
Step 1: Solution A is cooled and becomes white as solid B precipitates out of it.
Step 2: Solid B is collected by filtration and dried.
Step 3: Solid B is heated until it decomposes into solid C and gas D.
Step 4: Solid C and liquid E mix homogeneously.
Step 5: Gas D is found not to be flammable in air.
Question 25.
Which of the following conclusions can be drawn from these observations?
- Solution A is an acid.
- Solid B is an element.
- Solid C is a compound.
- Gas D is a pure substance.
Question 26.
In which of the following pairs of steps are both processes physical?
- step 1 and step 2
- step 1 and step 5
- step 2 and step 3
- step 3 and step 5
Question 27.
Use the procedural steps below to answer the question that follows.
- Compare results of calculations to known values.
- Identify the unknown metals.
- Calculate the density of the unknown metal.
- Record the mass of the unknown metal sample.
- Arrange the metals from least to most dense.
- Determine the volume of the unknown metal sample using water displacement.
Students are conducting an investigation on several metals in the same group in the periodic table. They are provided the density of one of the metals within the group. They are then given three unidentified metal samples and must identify each sample. In which of the following orders should the students follow the procedural steps shown?
- 4, 6, 1, 4, 5, 2
- 5, 1, 4, 6, 2, 3
- 6, 4, 3, 2, 1, 5
- 4, 6, 3, 5, 1, 2
Question 28.
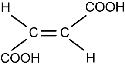
Use the table below to answer the question that follows.
| Name | Image | Melting Point | Density |
|---|---|---|---|
| maleic acid | structural chemical formula: Two Cs are connected by double horizontal lines. Each C is also connected to two single horizontal lines. The upper line on the left C connects to C O O H. The lower line on the left C connects to H. The upper line on the right C connects to C O O H. The lower line on the right C connects to H. | 135°C | 1.58 g/m3 |
| fumaric acid | structural chemical formula: Two Cs are connected by double horizontal lines. Each C is also connected to two single horizontal lines. The upper line on the left C connects to H. The lower line on the left C connects to C O O H. The upper line on the right C connects to C O O H. The lower line on the right C connects to H. | 287°C | 1.64 g/m3 |
Which of the following statements explains why maleic acid has a lower density and melting point than fumaric acid?
- Weaker dispersion forces are the main intermolecular force in maleic acid, because it is a nonpolar molecule.
- Maleic acid is more polar, resulting in stronger dipole-dipole attractions between molecules.
- Repulsion between carboxyl groups in fumaric acid causes the double bond to bend slightly, increasing intermolecular distance.
- The location of carboxyl groups in fumaric acid increases their ability to form hydrogen bonds between molecules.
Question 29.
Which of the following mixtures is the best example of a solution?
- air at sea level
- fog over a city street
- whipped cream
- wood fire smoke
Question 30.
A chemist hypothesizes that a newly developed photodegradable plastic will degrade twice as fast as currently available photodegradable plastics. Which of the following investigations would best test this hypothesis?
- modeling the chemical reactions involved in the degradation of the new plastic and using this model to project degradation rates under different environmental conditions
- subjecting the new plastic and the existing plastics to the same environmental conditions and measuring their degradation rates
- comparing the degradation rate of the new plastic with the degradation rates provided by the manufacturers of the existing photodegradable plastics
- exposing the new photodegradable plastic to conditions of constant UV radiation and no UV radiation and comparing the resulting degradation rates
Question 31.
Use the table below to answer the question that follows.
Properties of Selected Alcohols
| Alcohol | Melting Point (°C) |
Boiling Point (°C) |
Density (g/m3) |
|---|---|---|---|
| 2-propanol | minus 90 | 82 | 0.786 |
| cyclopentanol | minus 19 | 141 | 0.948 |
| cyclohexanol | 25 | 161 | 0.962 |
| 2-methyl-2-propanol | 26 | 82 | 0.789 |
An unknown alcohol is a liquid at room temperature (20°C), and a 50.0 mL sample has a mass of 39.4 g. Based on these properties, the unknown alcohol can be identified as:
- 2-propanol.
- cyclopentanol.
- cyclohexanol.
- 2-methyl-2-propanol.
Question 32.
Use the chemical equation below to answer the question that follows.
N A O H plus H N O 3 yields N A N O 3 plus H 2 O
Which of the following types of reactions is represented by the equation?
- redox reaction
- combustion reaction
- precipitation reaction
- neutralization reaction
Question 33.
Use the chemical equations below to answer the question that follows.
2 V s plus 5 F 2 g yields 2 V F 5 L
4 V s plus 5 O 2 g yields 2 V 2 O 5 S
2 V s plus N 2 g yields 2 V N S
The vanadium compound synthesis reactions shown can also be characterized as which of the following types of reaction?
- oxidation reactions
- redox reactions
- electrochemical reactions
- single replacement reactions
Question 34.
Use the chemical equation below to answer the question that follows.
Aqueous P B N O 3 2 plus 2 aqueous K B R yields 2 aqueous K N O 3 plus solid P B B R 2
The equation above shows a double replacement reaction. Which of the following equations represents the balanced net ionic equation?
- Aqueous P B N O 3 2 plus 2 aqueous B R minus yields 2 aqueous N O 3 minus plus solid P B B R 2
- Aqueous N O 3 minus plus aqueous K B R yields aqueous K N O 3 plus solid B R minus
- Aqueous K plus plus aqueous N O 3 minus yields aqueous K N O 3
- Aqueous P B 2 plus plus 2 aqueous B R minus yields solid P B B R 2
Question 35.
Use the chemical equation below to answer the question that follows.
aqueous M G C H 3 C O O 2 plus 2 aqueous K O H yields solid M G O H 2 plus 2 aqueous K C H 3 C O O
Aqueous solutions of magnesium acetate and potassium hydroxide react according to the equation shown. What is the driving force for this reaction?
- electron transfer
- neutralization
- ion transfer
- precipitation
Question 36.
In a reaction between KBr and P b N O 3 2 , which of the following substances will precipitate out of solution?
- P b Br 2
- K 2 P b
- K N O 3
- N O 3 B r
Question 37.
Use the unbalanced chemical equation below to answer the question that follows.
C 7 H 16 plus O 2 yields C O 2 plus H 2 O
What is the coefficient for the reactant O2 in the balanced equation for this reaction?
- 3
- 7
- 11
- 15
Question 38.
How many grams of carbon dioxide ( C O 2 ) can theoretically be produced by the complete combustion of 5.0 g butane ( C 4 H 10 ) in excess oxygen (O2)?
- 1.5 g
- 3.8 g
- 15 g
- 38 g
Question 39.
If a student requires 37.2 mL of 0.50 M N a O H to completely titrate 51.0 mL of HCl, what is the concentration of the HCl?
- 0.010 M
- 0.36 M
- 0.69 M
- 1.2 M
Question 40.
Use the information below to answer the question that follows.
Students performed an acid-base titration experiment. Student groups were asked to perform at least three trials in which sodium hydroxide stock solution was dispensed from a burette into a beaker of dilute hydrochloric acid and indicator until a color change was observed. The table below represents the data collected by one group during the experiment.
| Trial | Initial Burette Reading (mL) | Final Burette Reading (mL) | Volume Added (mL) |
|---|---|---|---|
| 1 | 0.00 | 12.53 | 12.51 |
| 2 | 2.50 | 15.12 | 12.69 |
| 3 | 0.00 | 27.75 | 27.73 |
Which of the following experimental errors most likely accounts for the data collected by this group during trial 3?
- misjudging the color of the indicator
- reading the burette volume from a different relative position
- incorrectly noting the initial burette reading
- reversing the position of the acid and base in the experimental setup
Question 41.
Which of the following chemical equations is balanced correctly?
- Solid Z N plus 2 aqueous A G N O 3 yields 2 solid A G plus aqueous Z N N O 3 2
- liquid C H 3 O H plus 2 gaseous O 2 yield gaseous C O 2 plus 2 liquid H 2 O
- solid A L plus 3 gaseous O 2 yields solid A L 2 O 3
- Aqueous H 2 S O 4 plus 2 aqueous N A O H yield solid N A 2 S O 4 plus liquid H 2 O
Question 42.
Use the chemical equation below to answer the question that follows.
2 liquid H 2 O 2 yield 2 liquid H 2 O plus gaseous O 2
Hydrogen peroxide decomposes according to the equation shown. What volume of oxygen gas, measured at standard temperature and pressure, will be produced from the decomposition of 50.0 g of hydrogen peroxide?
- 16.5 L
- 23.5 L
- 33.0 L
- 65.9 L
Question 43.
The protein elastin has the molecular formula C 27 H 48 N 6 O 6 . Which of the following elements makes up the smallest percentage of elastin by mass?
- C
- H
- N
- O
Question 44.
Elemental analysis of an unknown iron oxide sample showed it to be 77.7% iron by mass. What is the empirical formula of this unknown oxide?
- F e O
- F e 2 O 3
- F e 3 O 4
- F e 4 O 5
Question 45.
What is the molar mass of the compound Zr N O 3 4 ?
- 259.2 g/mol
- 297.2 g/mol
- 339.2 g/mol
- 612.8 g/mol
Question 46.
What is the percentage by mass of oxygen in C 3 H 5 N 3 O 9 ?
- 7.045%
- 37.18%
- 63.41%
- 84.20%
Question 47.
A compound is found to be composed of 30.9% sodium, 47.7% chlorine, and 21.5% oxygen. What is the empirical formula of this compound?
- N a C l O
- N a C l O 2
- N a C l O 3
- N a C l O 4
Question 48.
A substance with an empirical formula of CH2 has a molecular weight of 28.05 amu. What is the molecular formula for this substance?
- CH
- CH2
- C2H4
- C3H6
Question 49.
What is the pH of the resulting solution when 45.0 mL of 1.20 M lactic acid (HC3H5O3, K sub A equals 1 point 3 8 times 10 to the negative fourth power ) is fully neutralized through reaction with 30.0 mL of 1.80 M potassium hydroxide ( K O H )?
- 5.14
- 7.00
- 8.86
- 13.86
Question 50.
Use the graph below to answer the question that follows.
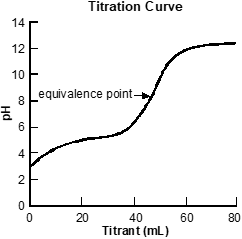
A set of x y axes is shown, with the title Titration Curve. The x axis is labeled Titrant milliliters and extends from 0 to 80. The y axis is labeled pH and extends from 0 to 14. A line curves up from x equals 0 y equals 3, becoming horizontal at x equals 30 y equals 5. The line increases sharply until x equals 60 y equals 12 before gradually becoming horizontal again. X equals 50 y equals 8 is labeled equivalence point.
The graph above depicts the results of a titration experiment. Which of the following conclusions about the titration experiment is best supported by these data?
- The acid being titrated is a polyprotic acid.
- A strong base is being titrated with a weak acid.
- The titrant used in this experiment is a strong acid.
- A weak acid is being titrated with a strong base.
Question 51.
What is the pH of a buffer made from 0 point 2 5 molar N H 3 and 0 point 5 0 molar N H 4 C L at 25 degrees Celsius, left parenthesis K sub b for N H 3 equals 1 point 8 times 10 to the negative fifth power right parenthesis ?
- 4.7
- 7.5
- 9.0
- 13
Question 52.
A chemist uses 22.0 mL of 0.10 M H 2 S O 4 to neutralize 10.0 mL of N a O H . What is the concentration of the N a O H solution?
- 0.055 M
- 0.11 M
- 0.22 M
- 0.44 M
Question 53.
How many grams of N a C l are contained in 50.0 mL of a 1.75 M N a C l solution?
- 35.0 g
- 28.6 g
- 5.12 g
- 0.875 g
Question 54.
What is the pH of a 0.22 M hypochlorous acid ( H O C l ) solution? ( K sub a equals 3.5 times 10 to the negative 7 power )
- 0.66
- 3.56
- 5.80
- 6.46
Question 55.
Assuming complete dissociation of the solute, what is the freezing point of a solution containing 24.0 g of SrCl2 and 100.0 g of water?
- negative 2.32°C
- negative 2.81°C
- negative 5.62°C
- negative 8.44°C
Question 56.
Assuming complete dissociation of electrolytes, which of the following solutions would have the lowest boiling point?
- 120.0 g of C 6 H 12 O 6 in 1.0 Liter of H 2 O
- 100.0 g of C 3 H 8 O 3 in 1.0 Liter of H 2 O
- 80.0 g of Zn S O 4 in 1.0 L of H 2 O
- 60.0 g of N H 4 C l in 1.0 Liter of H 2 O
Question 57.
Use the graph below to answer the question that follows.
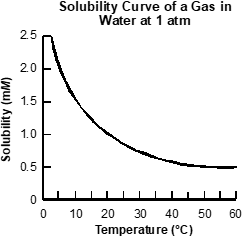
An x y axis is shown, with the title solubility curve of a gas in water at 1 atmosphere. The x axis is labeled temperature in degrees Celsius and extends from 0 to 60. The y axis is labeled solubility in milli moles per liter and extends from 0 to 2.5. A concave down curved line extends from x equals 0 y equals 2.5 to x equals 60 y equals 0.5.
Based on the gas solubility curve shown, which of the following changes will cause gas to be evolved?
- cooling a 0.50 mM solution from 25°C to 10°C at constant pressure
- warming a 2.0 mM solution from 5°C to 20°C at constant pressure
- cooling a 1.5 mM solution from 10°C to 5°C at constant pressure
- warming a 1.0 mM solution from 5°C to 15°C at constant pressure
Question 58.
Which of the following statements explains the differences in properties between ice and liquid water?
- H 2 O molecules in solid phase have no fixed volume but are spread far apart in constant, random movement.
- H 2 O molecules in liquid phase have no fixed volume but are spread far apart in constant, random movement.
- H 2 O molecules in liquid phase are in an ordered arrangement of a fixed position with small vibrations.
- H 2 O molecules in solid phase are in an ordered arrangement of a fixed position with small vibrations.
Question 59.
Use the graph below to answer the question that follows.
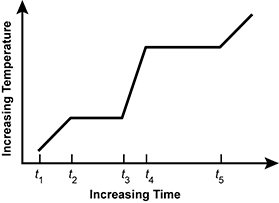
An x y axis is shown, with the x axis labeled increasing time and the y axis labeled increasing temperature. Five points: t 1, t 2, t 3, t 4, and t 5 are labeled on the x axis. Between t 1 and t 2, temperature increases. Between t 2 and t 3, temperature does not increase. Between t 3 and t 4, temperature increases. Between t 4 and t 5, temperature does not increase. After t 5, temperature increases.
The graph represents a pure material that is heated at a constant rate and goes from a solid to a liquid and then from a liquid to a gas under normal atmospheric pressure. Which of the following time intervals represents a liquid-gas phase transition?
- t 1 to t 2
- t 2 to t 3
- t 3 to t 5
- t 4 to t 5
Question 60.
Use the diagram below to answer the question that follows.
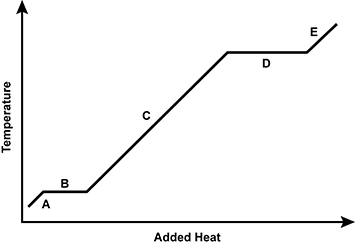
An x y axis is shown, with the x axis labeled added heat and the y axis labeled temperature. The graph alternates between increasing and horizontal sections, labeled A, B, C, D, and E.
Which of the following regions of the heating curve is accurately paired with a description of what is taking place at the molecular level?
- Region E: breaking molecular interactions in a gas
- Region B: increasing molecular kinetic energy in a solid
- Region C: increasing molecular kinetic energy of a liquid
- Region A: breaking intermolecular interactions in a solid
Question 61.
A 1.25 L balloon is taken from a location where it was held at 273.00 K and 1.00 atm to the top of a mountain where the pressure is 0.330 atm and the temperature is 259.11 K. What is the volume of the balloon at the mountain's summit?
- 0.435 L
- 0.750 L
- 3.59 L
- 3.99 L
Question 62.
The constituent particles in a substance in its solid state tend to:
- remain motionless.
- vibrate about fixed positions.
- slide freely past one another.
- move constantly in straight lines.
Question 63.
Use the phase diagram below to answer the question that follows.
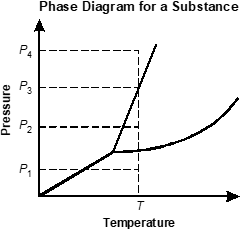
An x y axis is shown, titled Phase diagram for a substance. The x axis is labeled temperature. The y axis is labeled pressure. An increasing line extends from x equals 0 y equals 0 before splitting into a y shape. A dotted line extends vertically from an x axis value after the y shaped split, labeled T. A point in the lower region is labeled P 1. A point in the middle region is labeled P 2. A point on the line between the middle and upper regions is labeled P 3. A point in the upper region is labeled P 4.
Based on the phase diagram shown, which of the following pressures in combination with temperature T would result in the substance being present only as a gas?
- P sub 1
- P sub 2
- P sub 3
- P sub 4
Question 64.
A 20.0 L cylinder of oxygen gas is at a temperature of 27.0°C and a pressure of 5.00 atm. What is the density of the oxygen gas in the cylinder?
- 72.0 g/L
- 6.50 g/L
- 3.25 g/L
- 0.203 g/L
Question 65.
Which of the following statements best explains why ethanol ( C H 3 C H 2 O H ) completely dissolves in water, while ethane (CH3CH3) is largely insoluble in water?
- Ethanol is ionic and ethane is only slightly polar, so ethanol has stronger ion-dipole interactions with water.
- The molecular size of ethanol is larger than that of ethane, so the ethanol molecules are more readily dispersed in water.
- Ethanol is polar and ethane is nonpolar, so ethanol has stronger polar interactions with water.
- Intermolecular forces in ethanol are weaker than intermolecular forces in ethane, so ethanol is more readily dispersed in water.
Question 66.
For which of the following interactions is the ion-dipole force the strongest intermolecular force present?
- an interaction between two water ( H 2 O ) molecules
- an interaction between a lithium cation ( L i positive ) and a chloride anion ( C l negative ) in lithium chloride ( L i C l ) salt
- an interaction between a water ( H 2 O ) molecule and sodium ( N a positive ) cation in aqueous sodium chloride solution
- an interaction between an octane (C8H18) molecule and a decane (C10H22) molecule in a mixed hydrocarbon liquid, such as gasoline
Question 67.
The magnitude of van der Waals forces increases with:
- decreasing atomic radii.
- decreasing surface area of molecules.
- increasing polarizability due to electrons.
- increasing branching in molecular chains.
Question 68.
Which of the following molecules can form a hydrogen bond with water?
- HCl
- H2S
- CH3F
- NH3
Question 69.
In which of the following substances are dipole-dipole forces the primary intermolecular force?
- PCl5
- CCl4
- B e C l 2
- NCl3
Question 70.
Methanol is considerably more soluble in water than 1-hexanol. Which of the following best explains this difference in solubility?
- Methanol contains fewer hydroxyl groups than 1-hexanol.
- The alkyl group is longer in 1-hexanol than in methanol.
- 1-hexanol is a significantly more polar molecule than methanol.
- The greater number of hydrogen atoms in 1-hexanol increases the amount of hydrogen bonding.
Question 71.
Use the chemical equation below to answer the question that follows.
Solid N H 4 H S yields gaseous H 2 S plus gasesous N H 3
At a particular temperature, ammonium bisulfide (NH4HS) decomposes to form hydrogen sulfide (H2S) and ammonia (NH3) and comes to equilibrium in a container where both concentrations are 0.261 M. What is the value of the equilibrium constant, K sub e q , at the given temperature?
- 0.068
- 0.100
- 0.531
- 0.675
Question 72.
Use the chemical equation below to answer the question that follows.
2 gaseous H I yields gaseous H 2 plus gaseous I 2
A closed 1 L vessel contains 2 mol H I . The H I partially decomposes into H2 and I2 according to the equation shown. The equilibrium constant for the reaction at the given temperature is 0.02. If x represents the equilibrium concentration of H2, which of the following equations can be used to calculate x?
- 0.02 = x over the quantity 2 minus x
- 0.02 = x divided by the squared quantity 2 minus x
- 0.02 = x squared divided by the quantity 2 minus x
- 0.02 = x squared divided by the squared quantity 2 minus 2 x
Question 73.
At a given instant, the reaction quotient (Q) for a reversible reaction is calculated as 0.74 and the equilibrium constant ( K sub E Q ) is 14.5. Which of the following claims is accurate based on these experimental calculations?
- The reaction mixture is at equilibrium.
- The reaction mixture has only products.
- The rate of the forward reaction is faster.
- The rate of the reverse reaction is faster.
Question 74.
Which of the following statements is true for a buffer mixture made up of 250 mL of 0.1 M acetic acid and 125 mL of 0.1 M sodium acetate solution?
- Addition of HCl will decrease the equilibrium constant of the system.
- The pH value of the mixture will remain constant after an increase in temperature.
- The equilibrium constant will remain the same if the buffer mixture is diluted.
- At equilibrium, the species in the mixture are acetic acid molecules and acetate ions.
Question 75.
Use the chemical equation below to answer the question that follows.
2 gaseous C O 2 yield 2 gaseous C O plus gaseous O 2
Which of the following changes would affect the value of the equilibrium constant for the reaction shown?
- increasing the pressure
- removing O2 as it is formed
- adding a catalyst to the reaction
- decreasing the temperature
Question 76.
Use the chemical equation below to answer the question that follows.
2 gaseous N 2 plus 6 liquid H 2 O yield 4 gaseous N H 3 plus 3 gaseous O 2. delta H equals 1,530 kilojoules
Which of the following changes would cause the equilibrium reaction shown to shift to the right?
- removing N H 3 gaseous
- increasing the pressure
- decreasing the temperature
- adding H 2 O liquid
Question 77.
A student measures the rate of a chemical reaction between two aqueous solutions. Which of the following changes the student could make would be most likely to increase the rate of reaction between the compounds in solution?
- decreasing the temperature of the solutions so that the particles are less likely to fly past each other
- grinding each solute to a powder before dissolving it to increase the surface area and allow more particles to interact
- mixing a larger volume of each solution so that there are more total particles available to collide with each other
- increasing the concentration of the solutions so that the particles are more likely to encounter each other
Question 78.
Use the information below to answer the question that follows.
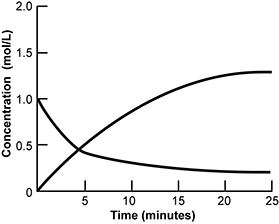
An x y axis is shown, with the x axis labeled time in minutes and extends from 0 to 25. The y axis is labeled concentration in moles per liter and extends from 0 to 2. A concave down line extends from x equals 0 y equals 0 until it becomes horizontal at x equals 25 y equals 1.5. A separate concave down line extends from x equals 0 y equals 1 until it becomes horizontal at x equals 25 y equals 0.25.
The graph represents the concentration versus time for N O 2 and N2O4 gases during the following chemical reaction.
2 gaseous N O 2 yield gaseous N 2 O 4
Which of the following conclusions about this chemical reaction is best supported by the data provided?
- Equilibrium is established at about t = 5 minutes.
- The speed of the forward reaction is equal to the speed of the reverse reaction at about t = 25 minutes.
- The rate law of the reaction is first order with respect to each molecule.
- The activation energy of the forward reaction is greater than that of the reverse direction.
Question 79.
Use the information below to answer the question that follows.
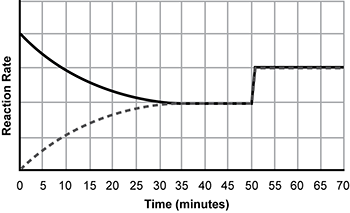
An x y axis is shown. The x axis is labeled time in minutes and extends from 0 to 70. The y axis is labeled reaction rate and extends from 0 to 5. A dotted line increases from x equals 0 y equals 0 and a solid line decreases from x equals 0 y equals 4 until the lines meet at x equals 35 y equals 2. The combined lines extend horizontally until x equals 50 before increasing vertically to y equals 3, before continuing horizontally.
The graph shows the forward and reverse rates of reaction for the reversible gas phase reaction between carbon monoxide C O and chlorine Cl2 shown below.
gaseous C O plus gaseous C L 2 yield gaseous C O C L 2. delta H sub R X N equals negative 163 kilojoules per mole
Which of the following changes to the system would most likely cause the effect observed at time t = 50 minutes?
- an increase in temperature
- an increase in pressure
- use of a catalyst
- change in surface area
Question 80.
Use the table below to answer the question that follows.
| Experiment | Initial [A2] (M) | Initial [B] (M) | Initial Rate (M/s) |
|---|---|---|---|
| 1 | 0.25 | 0.10 | 2.8 times 10 to the negative 2 power |
| 2 | 0.25 | 0.30 | 8.3 times 10 to the negative 2 power |
| 3 | 0.25 | 0.40 | 1.1 times 10 to the negative 1 power |
| 4 | 0.50 | 0.10 | 5.5 times 10 to the negative 2 power |
| 5 | 0.75 | 0.30 | 2.5 times 10 to the negative 1 power |
The table shows initial concentrations and reaction rates for the hypothetical reaction A 2 plus 2 B yields 2 A B Using these data, which of the following is the rate law for this reaction?
- rate = k times the concentration of A 2
- rate = k times the concentration of A 2 times the concentration of B squared
- rate = k times the concentration of A 2 times the concentration of B
- rate = k times the concentration of A 2 cubed times the concentration of B
Question 81.
Use the table below to answer the question that follows.
| Experiment | Initial [H2O2] (M) | Initial Rate (M/min.) |
|---|---|---|
| 1 | 1.50 times 10 to the negative 2 | 1.59 times 10 to the negative 5 |
| 2 | 3.00 times 10 to the negative 2 | 3.18 times 10 to the negative 5 |
| 3 | 4.50 times 10 to the negative 1 | 4.77 times 10 to the negative 5 |
| 4 | 7.50 times 10 to the negative 2 | 7.95 times 10 to the negative 5 |
Using the data for the decomposition of H2O2 shown, what is the reaction rate when The concentration of H 2 O 2 = 6.50 times 10 to the negative 1 ?
- 6.36 times 10 to the negative 5 M/min.
- 6.89 times 10 to the negative 4 M/min.
- 4.60 times 10 to the negative 2 M/min.
- 6.50 times 10 to the negative 1 M/min.
Question 82.
Use the information below to answer the question that follows.
The proposed mechanism for the reaction between N O 2 and C O at temperatures less than 600 K is shown below.
step 1: 2 gaseous N O 2 yield gaseous N O 3 plus gaseous N O slow. step 2: Gaseous N O 3 plus gaseous N O yield gaseous N O 2 plus gaseous C O 2 fast. overall: Gaseous N O 2 plus gaseous C O yield gaseous N O plus gaseous C O 2,
Given this information, which of the following rate laws best represents this reaction mechanism?
- rate = k times the concentration of C O
- rate = k times the concentration of N O 2 squared
- rate = k times the concentration of N O 3 times the concentration of C O
- rate = k times the concentration of N O 2 squared times the concentration of C O
Use the information below to answer the two questions that follow.
Students are using the experimental setup shown below to investigate the enthalpy change of the combustion of ethanol ( C 2 H 5 O H ). The students calculated the enthalpy change of the combustion reaction as negative 945 kJ/mol of ethanol.
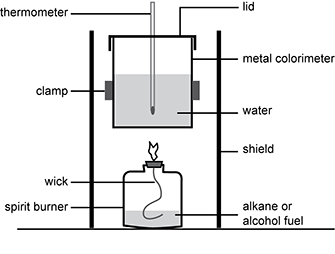
A spirit burner is half-filled with alkane or alcohol fuel. A flaming wick extends from the burner. A metal calorimeter is filled with water, into which a thermometer is placed. A lid covers the calorimeter. The calorimeter is held over the burner by a clamp and surrounded by a shield.
Question 83.
The accepted experimental enthalpy change of the reaction is negative 1368 kJ/mol. Which of the following experimental modifications would most likely decrease the percent error?
- decreasing the number of data points used in the calculation
- decreasing the heat loss from the calorimeter to the environment
- increasing the precision of the thermometer
- increasing the amount of ethanol used in the spirit burner
Question 84.
If ethanol ( C 2 H 5 O H ) in the burner is replaced with the same number of moles of 1-pentanol ( C 2 H 11 O H ), which of the following statements will be true?
- The measured temperature difference will remain the same.
- The percent error of the experiment will be decreased.
- More energy will be released.
- The fuel will burn for a longer time.
Question 85.
For which of the following reactions would delta S be positive?
- Liquid H 2 O yields solid H 2 O
- Gaseous H 2 plus gaseous I 2 yield 2 gaseous H I
- Aqueous A G plus plus aqueous C L minus yield solid A g C L
- Solid C 6 H 12 O 2 plus 6 gaseous O 2 yield 6 gaseous C O 2 plus 6 gaseous H 2 O
Question 86.
Given that the specific heat of copper is 0.385 J/(g•K), how much heat is required to raise the temperature of 50.0 g of copper from 25.0°C to 100°C?
- 1.44 kJ
- 1.93 kJ
- 6.70 kJ
- 7.18 kJ
Question 87.
When 1.0 times 10 squared g of an unknown metal at 80.0°C is placed in a calorimeter containing 1.0 times 10 squared g of water, the temperature of the water rises from 20.0°C to 25.0°C. Given that the specific heat of water is 4.184 J/(g•K), what is the specific heat of the metal?
- 0.26 J/(g•K)
- 0.38 J/(g•K)
- 1.52 J/(g•K)
- 1.90 J/(g•K)
Question 88.
A constant pressure calorimeter with negligible heat capacity contains 200.0 g of H 2 O at 25.00°C. When 12.3 g of K C l O 3 is dissolved in the H 2 O , the temperature of the solution in the calorimeter drops to 20.05°C. Assuming that the specific heat of the solution is 4.184 J/(g•K), what is the heat of solution of K C l O 3 ?
- 0.255 kJ/mol
- 0.337 kJ/mol
- 44.0 kJ/mol
- 168 kJ/mol
Question 89.
A reaction at 750 K has a Delta H ° = negative 150 kJ/mol, and a Delta S ° = +200 J/(mol•K). Which of the following statements is true?
- The reaction is spontaneous at all temperatures.
- The reaction is spontaneous at all temperatures above 750 K.
- The reaction is not spontaneous at any temperatures.
- The reaction is spontaneous at all temperatures below 750 K.
Question 90.
Given the relationship between standard free energy change ( Delta G °) and the equilibrium constant (K), which of the following claims is true when K = 6 times 10 to the fifth power at a particular temperature T?
- The equilibrium favors products, and decreasing temperature will decrease K.
- Delta G ° is less than zero, and the equilibrium favors products.
- The equilibrium favors reactants, and increasing temperature will increase K.
- Delta G ° is greater than zero, and the equilibrium favors reactants.
Question 91.
Use the table and chemical equation below to answer the question that follows.
| Bond | Bond Enthalpy kJ/mol |
|---|---|
| H to H | 432 |
| H to I | 295 |
gaseous H 2 plus gaseous I 2 yield 2 gaseous H I. delta H equals negative 9 kiloJoules per mole
Based on the bond enthalpies and chemical equation shown, what is the best estimate for the I to I bond in the equation?
- Negative 286 kJ/mol
- Negative 9 kJ/mol
- 149 kJ/mol
- 286 kJ/mol
Question 92.
Use the table and chemical equation below to answer the question that follows.
| Bond | Bond Enthalpy kJ/mol |
|---|---|
| O is connected to O by a single horizontal line | 138 |
| O is connected to O by a double horizontal line | 498 |
| Cl is connected to Cl by a single horizontal line | 243 |
1 half O 2 gaseous H + C l 2 gaseous yield O C l 2 gaseous
The standard enthalpy of formation of O C l 2 is 105 kJ/mol. Based on the bond enthalpies and chemical equation shown, what is the best estimate for the bond enthalpy for an O to Cl bond?
- 104 kJ/mol
- 194 kJ/mol
- 318 kJ/mol
- 387 kJ/mol
Question 93.
The bond energy for oxygen gas (O2) is 499 kJ/mol. Based on this information, what is the standard enthalpy of formation of gaseous oxygen atoms (O)?
- 0.00 kJ/mol
- 250 kJ/mol
- 499 kJ/mol
- 998 kJ/mol
Question 94.
Use the table below to answer the question that follows.
| Reaction | Chemical Equation | standard delta H in kJ | standard delta S in J/K |
|---|---|---|---|
| 1 | 2 gaseous S O 3 yield 2 gaseous S O 2 plus gaseous O 2 | 198 | 188 |
| 2 | 2 liquid A S F 3 yield 2 solid A S plus 3 gaseous F 2 | negative 1643 | 316 |
| 3 | Gaseous N 2 O plus 2 liquid H 2 O yield solid H H 4 N O 3 | 36 | negative 446 |
| 4 | 4 solid F e + 3 gaseous O 2 yield 2 solid F e 2 O 3 | negative 1650 | negative 549 |
Which of the reactions shown in the table is spontaneous only at sufficiently high temperatures?
- reaction 1
- reaction 2
- reaction 3
- reaction 4
Question 95.
Use the diagram below to answer the question that follows.
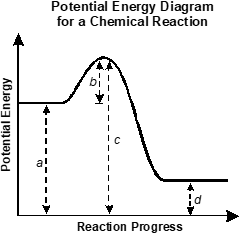
An x y axis is shown with the x axis labeled reaction progress and the y axis labeled potential energy. A horizontal line extends from reaction progress equals 0. The distance between the line and the x axis is labeled a. The line increases to a peak. The vertical distance between the horizontal segment of the line and its peak is labeled b. The distance between the peak and the x axis is labeled c. The line decreases before becoming horizontal again, at a lower level than the first horizontal section. The distance between the second horizontal section and the x axis is labeled d.
Which of the following expressions best represents the change in enthalpy for the reaction in the potential energy diagram shown?
- b minus c
- c minus b
- a minus d
- d minus a
Question 96.
Use the table below to answer the question that follows.
Standard Reduction Potentials
| Half-reaction | E standard in volts |
|---|---|
| K + + e negative yields K | negative 2.93 |
| Mg 2 + + 2 e negative yields Mg | negative 2.37 |
| A l 3 + + 3 e negative yields A l | negative 1.66 |
| Zn 2 + + 2 e negative yields Zn | negative 0.76 |
| Pb 2 + + 2 e negative yields Pb | negative 0.13 |
| Sn 2 + + 2 e negative yields Sn | +0.13 |
| Hg 2 + + 2 e negative yields Hg | +0.85 |
| Cl 2 + 2 e negative yields 2Cl negative | +1.36 |
| A u 3 + + 3 e negative yields A u | +1.50 |
An electrochemical cell is being designed for a particular application, and it needs to produce as closely as possible a voltage of 3.00 V. The substances listed in the table are available for the project. Which of the following pairs of half-cells will be the best choice to meet the specifications for the electrochemical cell?
- Pb pipe Pb 2 + and K pipe K +
- A l pipe A l 3+ and C L minus pipe C l 2
- Hg pipe Hg 2+ and A u pipe A u 3+
- Mg pipe Mg 2+ and Sn pipe Sn 2+
Use the information below to answer the two questions that follow.
| Trial | molar concentration of Z N 2 plus | Molar concentration of C U 2 plus | Log of the concentration C U 2 plus | Cell Potential (V) |
|---|---|---|---|---|
| 1 | 1.00 | 0.0010 | negative 3 | 0.950 |
| 2 | 1.00 | 0.010 | negative 2 | 0.920 |
| 3 | 1.00 | 0.10 | negative 1 | 1.05 |
| 4 | 1.00 | 1.0 | 0 | 1.10 |
aqueous C U 2 plus plus aqueous Z N yields solid C U plus aqueous Z N 2 plus E sub cell equals 1 point 1 0 volts.
Copper and zinc can undergo an electrochemical reaction according to the chemical equation shown. Cell potential (in volts) is measured during an experiment in which C u 2 + concentration is varied. Zn 2+ concentration is held relatively constant during these trials by reacting far more zinc than copper. The table represents these experimental data. The graph summarizes the relationship between copper ion concentration and cell potential.
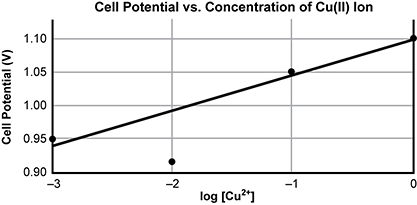
A scatter plot is shown, titled Cell Potential versus Concentration of C U 2 plus ion. The x axis is labeled log of the concentration of C U 2 + and extends from negative 3 to 0. The y axis is labeled cell potential in volts and extends from 0 point 9 0 to 1 point 1 0. Points exist at negative 3, 0 point 9 5, negative 2, 0 point 9 1, negative 1, 1 point 0 5, and 0, 1 point 1 0. A straight best fit line is drawn through the points, with the value at x equals negative 2 lying below the line.
Question 97.
Which of the following changes would most increase the precision of this experiment?
- replacing the voltmeter and rerunning the experiment
- repeating trial 2
- preparing a fresh stock solution with 1 M concentration
- conducting trials with concentrations below 0.005 M
Question 98.
Which of the following conclusions is best supported by the data shown in the table?
- Cell potential under nonstandard conditions is directly proportional to solution concentration.
- Cell potential depends on the concentration of the anode half-cell.
- The number of moles of electrons transferred between cells is constant.
- Decreasing the concentration of the cathode half-cell decreases the cell potential.
Question 99.
Use the table and diagram below to answer the question that follows.
Standard Reduction Potentials
| Half-reaction | E standard in volts |
|---|---|
| Zn 2 + + 2 e negative yields Zn | negative 0.76 |
| C u 2 + + 2 e negative yields C u | +0.34 |
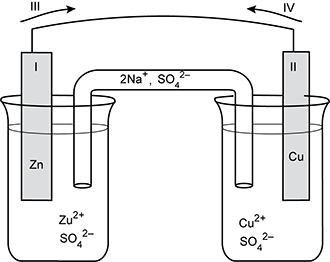
Two beakers are shown, connected by a salt bridge, on which the left side is labeled 2 N a + and the right side labeled S O 4 2 minus. The left beaker contains a solution labeled with Z u 2 + and S O 4 2 minus. An electrode labeled 1 and Z n extends up to a semicircular wire. An arrow labeled 3 points to the right. At the other end of the wire, an arrow labeled 4 points left. An electrode labeled 2 and Cu extends down into the right beaker, which contains a solution labeled C U 2 + and S O 4 2 minus.
Which of the following lists describes the characteristics of the voltaic cell shown in the diagram?
- 1 = anode, 2 = cathode, 3 = direction of electron flow
- 1 = anode, 2 = cathode, 4 = direction of electron flow
- 1 = cathode, 2 = anode, 3 = direction of electron flow
- 1 = cathode, 2 = anode, 4 = direction of electron flow
Question 100.
Use the table below to answer the question that follows.
Standard Reduction Potentials
1 point 0 molar at 25 degrees Celsius
| Half-reaction | E standard in (V) |
|---|---|
| aqueous B A 2 plus plus 2 e minus yields solid B A | negative 2.91 |
| aqueous N A plus plus e minus yields solid N A | negative 2.71 |
| aqueous M N 2 plus plus 2 e minus yields solid M N | negative 1.18 |
| Aqueous T L plus plus e minus yields solid T L | negative 0.34 |
| aqueous A G plus plus e minus yeidls solid A G | positive 0.80 |
| aqueous A U 3 plus plus 3 e minus yields solid A U | positive 1.50 |
Using the standard reduction potentials shown, which of the following cells is spontaneous at standard conditions?
- M N pipe M N 2 plus double pipe T L plus pipe T L
- A G pipe A G plus double pipe M N 2 plus pipe M N
- A G pipe A G plus double pipe N A plus pipe N A
- N A pipe N A plus double pipe B A 2 plus pipe B A
Open-Response Items
The directions shown below represent what you will see on the actual test. For the purposes of this practice test, you will be able to type your written responses in the boxes provided on the answer key.
This section of the test consists of two open-response item assignments. You will be asked to prepare a written response of approximately 150–300 words, or 1–2 pages, for each assignment.
Read the assignments carefully before you begin your responses. Think about how you will organize your responses. You may use the erasable sheet(s) to make notes, write an outline, or otherwise prepare your responses. However, your final response to each assignment must be either:
- typed into the on-screen response box,
- written on a response sheet and scanned using the scanner provided at your workstation, or
- provided using both the on-screen response box (for typed text) and a response sheet (for calculations or drawings) that you will scan using the scanner provided at your workstation.
Instructions for scanning your response sheet(s) are available by clicking the "Scanning Help" button at the top of the screen.
As a whole, your response to each assignment must demonstrate an understanding of the knowledge of the field. In your response to each assignment, you are expected to demonstrate the depth of your understanding of the subject area by applying your knowledge rather than by merely reciting factual information.
Your responses to the assignments will be evaluated based on the following criteria.
- Purpose: the extent to which the response achieves the purpose of the assignment
- Subject Knowledge: appropriateness and accuracy in the application of subject knowledge
- Support: quality and relevance of supporting evidence
- Rationale: soundness of argument and degree of understanding of the subject area
The open-response item assignments are intended to assess subject knowledge. Your responses must be communicated clearly enough to permit valid judgment of the evaluation criteria by scorers. Your responses should be written for an audience of educators in this field. The final version of each response should conform to the conventions of edited American English. Your responses should be your original work, written in your own words, and not copied or paraphrased from some other work.
Be sure to write about the assigned topics. Remember to review your work and make any changes you think will improve your responses.
Any time spent responding to an assignment, including scanning the response sheet(s), is part of your testing time. Monitor your time carefully. When your testing time expires, a pop-up message will appear on-screen indicating the conclusion of your test session. Only response sheets that are scanned before you end your test or before time has expired will be scored. Any response sheet that is not scanned before testing ends will NOT be scored.
Question 101.
Use the information below to complete the exercise that follows.
Phenomenon—A balloon is inflated with air and sealed. The mass of the inflated balloon is measured and recorded. The balloon is then placed in a freezer for an hour. When the sealed balloon is removed from the freezer, it appears partially deflated, but its mass remains the same.
Use your knowledge of the relationship of kinetic molecular theory to the gas laws to write a response of approximately 150–300 words, or 1–2 pages, in which you:
- describe the key scientific concepts related to the phenomenon presented to the depth of knowledge a student would need to master the concept of the relationship of kinetic molecular theory to the gas laws;
- include a representative graph, formula, and/or diagram with labels to model the situation as the balloon cooled in the freezer in terms of the kinetic molecular theory and/or the gas laws; and
- discuss how a chemistry teacher could use the specific science and engineering practice of "extending investigations" to help students understand phenomena related to the kinetic molecular theory and the gas laws.
Question 102.
Use the information below to complete the exercise that follows.
The freezing point of seawater of average salinity is about negative 2°C compared with 0°C for pure water.
Use your knowledge of chemistry to write a response of approximately 150–300 words, or 1–2 pages, in which you:
- form and describe a testable scientific claim that addresses how the freezing point of water is affected by solutes;
- describe a scientific investigation to test the proposed claim, including identifying variables, data to be collected, and any general lab safety considerations;
- explain how the collected data may provide evidence that supports or refutes the tested claim; and
- discuss how a chemistry teacher could use the science and engineering practice of "planning and carrying out investigations" to help students make sense of the effects of solutes on the freezing point of water.